
protons and neutrally charged neutrons. An atom's electrons revolve

protons/electrons: 2. # neutrons: 2. Atomic mass: 4.002602

electrons than protons. An ion is not neutral an so it is radioactif.

The electrons revolve around

All atoms have different numbers of protons, neutrons and electrons.

which in turn are made of protons, neutrons, and electrons, like so.

After recombination, the electrons and protons formed atoms.

Normaly the number of electrons is the same as the number of protons and the

An oxygen atom has 8 protons, 8 electrons, and 8 neutrons

It has the same number of electrons (3) as protons.
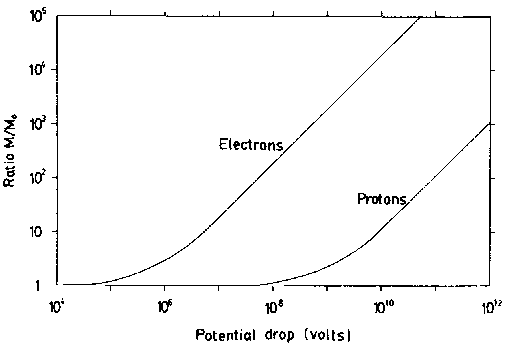
Mass Enhancement for Relativistic Electrons and Protons.

H protons neutrons electrons magnesium a sunday work on its valence shell

Energy and Electricity

As seen from the shadow of the Earth, electrons and protons are guided to
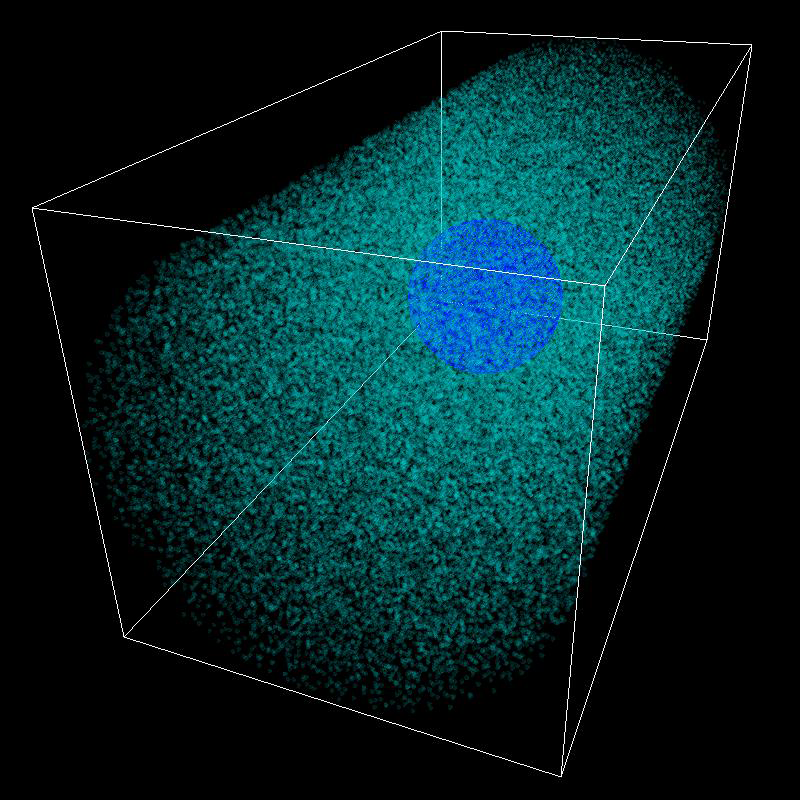
Electron Cloud simulation with electrons and protons rendered as particles.

protons, neutrons, electrons

As shown in Figure 3, ions with more electrons than protons are negatively

Model of a carbon atom showing the electrons, protons, and neutrons.

It has six protons, six neutrons and six electrons. It is an atom of carbon.
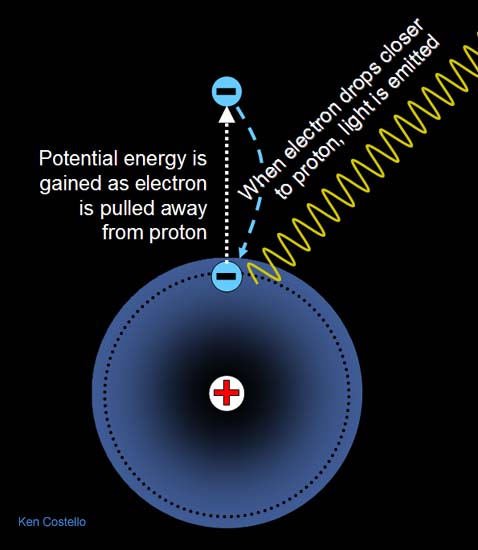
The proton is 2000 times heavier than the electron.